
Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Request PDF

Cationic palladium(II)–acetylacetonate complexes containing phosphine and aminophosphine ligands and their catalytic activities in telomerization of 1,3-butadiene with methanol - ScienceDirect
![Preparation and Application of Amino Phosphine Ligands Bearing Spiro[indane-1,2′-pyrrolidine] Backbone - J. Org. Chem. - X-MOL Preparation and Application of Amino Phosphine Ligands Bearing Spiro[indane-1,2′-pyrrolidine] Backbone - J. Org. Chem. - X-MOL](https://xpic.x-mol.com/20190712%2F10.1021_acs.joc.9b00875.jpg)
Preparation and Application of Amino Phosphine Ligands Bearing Spiro[indane-1,2′-pyrrolidine] Backbone - J. Org. Chem. - X-MOL

Scheme 1 Preparation of phosphinite functionalized silica palladium... | Download Scientific Diagram

PDF) Aminophosphine–palladium(II) complexes: Synthsesis, structure and applications in Suzuki and Heck cross-coupling reactions | Murat Aydemir - Academia.edu

Synthesis, structure, computational and catalytic activities of palladium complexes containing hydrazide based amino-phosphine ligands - ScienceDirect
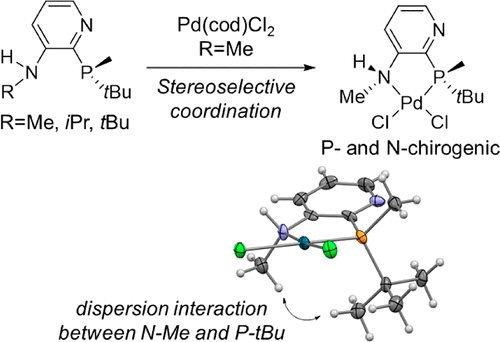
Development of P- and N-Chirogenic Ligands Based on Chiral Induction from a Phosphorus Donor to a Nitrogen Donor in Palladium Complexes - Organometallics - X-MOL

PDF) Aminophosphine Palladium Pincer-Catalyzed Carbonylative Sonogashira and Suzuki–Miyaura Cross-Coupling with High Catalytic Turnovers

Aminophosphine Palladium Pincer-Catalyzed Carbonylative Sonogashira and Suzuki-Miyaura Cross-Coupling with High Catalytic Turnovers. - Abstract - Europe PMC
![The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ... The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ...](https://onlinelibrary.wiley.com/cms/asset/16d0c16a-ffaf-4992-b9e6-91a5da65039b/mcontent.jpg)
The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ...
![Synthesis and characterization of palladium(II) complexes with chiral aminophosphine ligands: Catalytic behaviour in asymmetric hydrovinylation. Crystal structure of cis-[PdCl2(PPh((R)-NHCHCH3Ph)2)2] - ScienceDirect Synthesis and characterization of palladium(II) complexes with chiral aminophosphine ligands: Catalytic behaviour in asymmetric hydrovinylation. Crystal structure of cis-[PdCl2(PPh((R)-NHCHCH3Ph)2)2] - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022328X07001738-fx1.jpg)
Synthesis and characterization of palladium(II) complexes with chiral aminophosphine ligands: Catalytic behaviour in asymmetric hydrovinylation. Crystal structure of cis-[PdCl2(PPh((R)-NHCHCH3Ph)2)2] - ScienceDirect

Aminophosphine Palladium Pincer-Catalyzed Carbonylative Sonogashira and Suzuki-Miyaura Cross-Coupling with High Catalytic Turnovers. - Abstract - Europe PMC
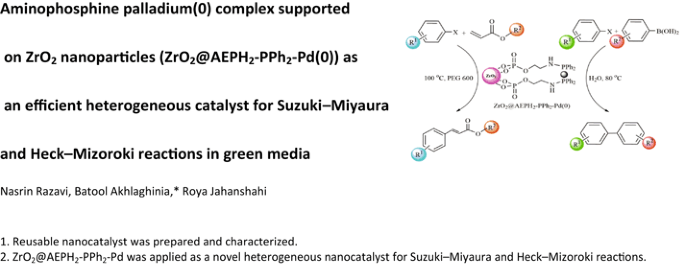
Aminophosphine Palladium(0) Complex Supported on ZrO 2 Nanoparticles (ZrO 2 @AEPH 2 -PPh 2 -Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | SpringerLink

Multifunctional chiral aminophosphines for enantiodivergent catalysis in a palladium‐catalyzed allylic alkylation reaction - Eliseenko - 2020 - Chirality - Wiley Online Library

Camphane-based aminophosphine ligands for Pd-catalyzed asymmetric allylic alkylation - ScienceDirect

Highly Convenient, Clean, Fast, and Reliable Sonogashira Coupling Reactions Promoted by Aminophosphine‐Based Pincer Complexes of Palladium Performed under Additive‐ and Amine‐Free Reaction Conditions - Bolliger - 2009 - Advanced Synthesis & Catalysis -

Table 1 from Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Semantic Scholar

Table 1 from Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Semantic Scholar

Synthesis, characterization, and application of palladium complexes containing bis(aminophosphine) ligands
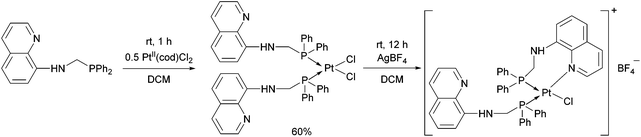
Synthesis of platinum, palladium and rhodium complexes of α-aminophosphine ligands - Dalton Transactions (RSC Publishing) DOI:10.1039/C8DT00178B

Bis-N,N-aminophosphine (PNP) crosslinked poly(p-tert-butyl styrene) particles: A new support for heterogeneous palladium catalysts for Suzuki coupling reactions - Catal. Commun. - X-MOL

Proposed mechanism for the palladium pincer-catalyzed carbonylative... | Download Scientific Diagram


