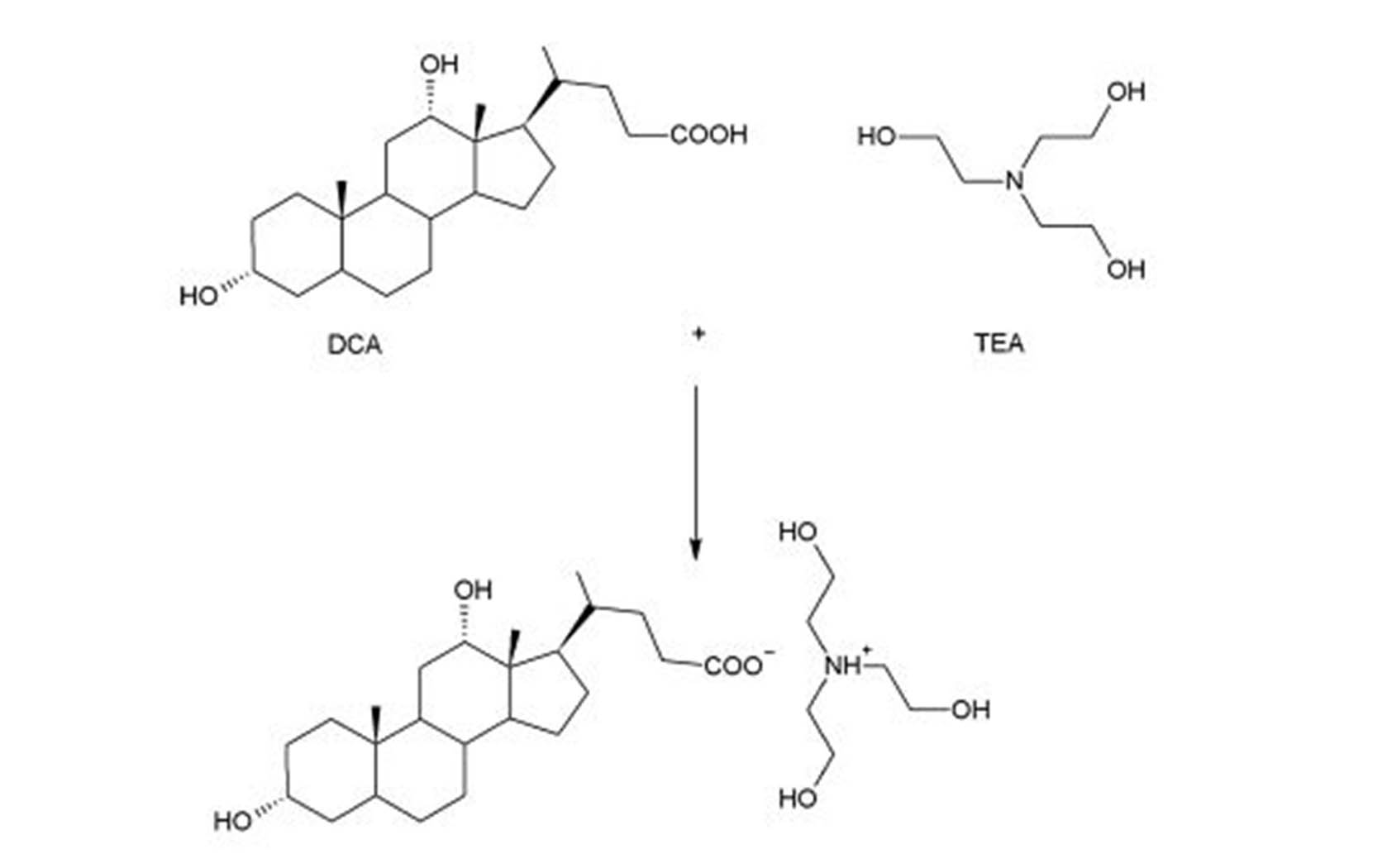
5. CV of 0.02M l-dopa in Triethanolamine buffer (pH 10), scan rate 50 mV/s | Download Scientific Diagram
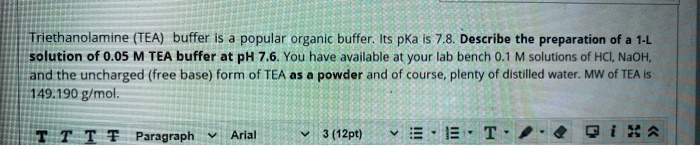
SOLVED: Triethanolamine (TEA) buffer Is popular organic buffer. Its pKa Is 7.8 Describe the preparation of a 1-L solution of 0.05 M TEA buffer at PH 7.6. You have availlable at your
![PDF] Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. | Semantic Scholar PDF] Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/dd0a648b5ba29c90ffad9154d9db8c3b5af10cfc/2-Table1-1.png)
PDF] Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. | Semantic Scholar

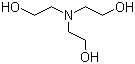
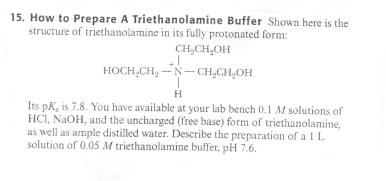
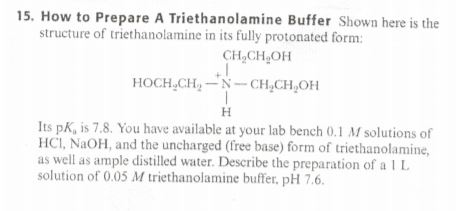
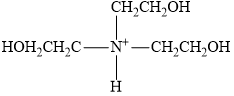
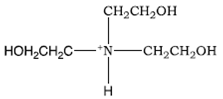
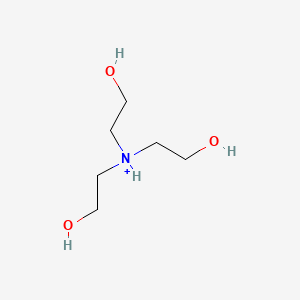
SOLVED: How to Prepare A Triethanolamine Buffer Shown hete is the structure Ol (riethanolamine in Its tully protonated orm; GH,CHLOH HOCH CH, CHCHOH Its pK, is 7. , You have available at